

Bonding molecular orbitals have lower energy than the atomic orbitals from which they were formed. If the atomic orbitals are combined with the same phase they interfere constructively and a bonding orbital is formed. The 2s orbitals combine primarily with each other to form another pair of bonding and antibonding orbitals at a higher energy.įigure 2 (below) shows how bonding and antibonding σ orbitals can be formed by combining s orbitals in-phase (bonding, bottom) and out-of-phase (antibonding, top). When Li 2 forms the two lowest energy orbitals are the pair of bonding and antibonding orbitals formed from the two possible combinations of the 1s on each atom. Molecular orbitals are best formed when composed of Atomic orbitals of like energies. Furthermore, all orbitals at an energy level must be filled with one electron before they can be paired. No more than 2 electrons can occupy 1 molecular orbital at a time. Antibonding orbitals are formed by out-of-phase combinations of atomic orbitals and decrease the electron density between atoms (see figure 2 below).įollowing both the Pauli exclusion principle and Hund's rule, electrons fill in orbitals of increasing energy.Įlectrons fill orbitals with the lowest energy first. Bonding orbitals are formed by in-phase combinations of atomic orbitals and increase the electron density between the atoms (see figure 2 below)Įlectrons in antibonding molecular orbitals cause a system to be destabilized since more energy is associated with bonded atoms than that of a system of unbound atoms. Both H atoms have a 1s orbital, so when bonded together, there are therefore two molecular orbitals.īonding molecular orbitals are lower energy than the atomic orbitals from which they were formed.Īntibonding molecular orbitals are higher energy than the atomic orbitals from which they were formed.Įlectrons in bonding molecular orbitals help stabilize a system of atoms since less energy is associated with bonded atoms as opposed to a system of unbound atoms. The molecule H 2 is composed of two H atoms. Total number of molecular orbitals is equal to the total number of atomic orbitals used to make them. The principles to apply when forming pictorial molecular orbitals from atomic orbitals are summarized in the table below: Principle Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the Pauli principle, the aufbau principle and Hund's rule just as with atoms.
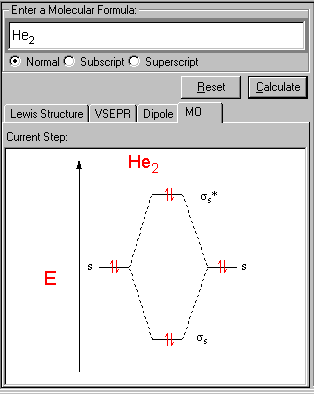
Each molecular orbital can only have 2 electrons, each with an opposite spin. Similar to atomic orbitals, molecular orbitals are wave functions giving the probability of finding an electron in certain regions of a molecule. In molecules, atomic orbitals combine to form molecular orbitals which surround the molecule.


 0 kommentar(er)
0 kommentar(er)
